Instruction for use
Trade name:Dermaxgel TM
Generic name:Modified Sodium Hyaluronate Gel For Injection
CONTENT
Stabilized hyaluronic acid = 20mg/ml; Sodium chloride physiological solution q.s; PH 7
DESCRIPTION
It is a sterile pyrogen-free physiological gel of crosslinked hyaluronic acid, of non-animal origin.
It is a colorless, odorless and highly viscous aqueous gel. It is supplied in a glass syringe with a luer-lock fitting. The contents of the syringe.Have been sterilized using moist heat. The product is for single use only. Disposable sterile Needles are provided with each syringe.Information about the sterilization method and size of the needle is printed on its packaging. The number of units per package and the volume contained in each syringe is as started on the outer package.
INTENDED USE
It is intended to be used for intradermal implantation to correct defect of soft tissue after accidents and trauma.
STERILIZATION
The syringe containing is sterilized by steam. The needle is sterilized by means of gama radiation
MODE OF ACTION
This product is a filler that adds volume to the tissue. The volume and the lifting capacity originate from the ability of.The hyaluronic acid to attract high amount of water, Which is further increased by the stabilization process.It will in time undergo isovolemic degradation, Which means that the product maintains its Volume even during degradation.
INDICATION
It is indicated for soft tissue augmentation. The depth of injection is the dermal layer of the skin.For facial areas with limited soft tissue support and soft tissue cover,e.g.the periorbital Region, injection into the subcutaneous fatty tissue or supraperiostal administration are recommended.
WARNING
The product is only intended for use as an intradermal implant. Confirm that the product has not expired and that sterility has not been compromised before use, product is for single use only; do not re-use. In case of re-use, this can cause to depress performance of the product and may lead to severe cross-infection.Used needles and syringes must be disposed into a llector designated for this purpose.
Do not inject intravascularly. As for other injectable medical devices, inadvertent injection into blood vessels could potentially lead to vascular occlusion, ischemia and necrosis. Aspiration prior to injection is recommended.
If blanching is observed, i.e. the overlying skin turns a whitish colour, the injection should be stopped at once.
And the area massaged until it returns to a normal colour.
Do not use in patients with bleeding disorders or in patients who are taking thrombolytics or anticoagulants.
Do not resterilize gel.
Do not mix with other products prior to injection of the device.
ADVERSE REACTIONS
Patients must be informed of the potential risks and adverse events related to the injection procedure and to the use of this product. A slight bleeding may occur during the injection,and it disappears spontaneously as soon as the injection is finished. In occasional cases one or more of the following may occur either immediately or as a delayed reaction (list not exhaustive) Reactions usually associated with injections such as redness, erythema, oedema or pain sometimes accompanied by itching in the treated area. These reactions may last for a week.Hematomas in the treated area,Swelling in the treated area,Indurations or nodules in the treated area, Coloration or discoloration in the treated area,
Allergy to one of the product’s components, especially to sodium hyaluronate.Cases of necrosis,abscesses and granulomas after sodium hyaluronate injections have been reported in the literature. These rare potential risks must nevertheless be considered.
Patients should be instructed to report any side effects which last for more than one week to his/her practitioner. The practitioner may then prescribe the patient appropriate treatment.
Any other undesirable side effects associated with injection of the product must be reported to the distributor and/or to the manufacturer.
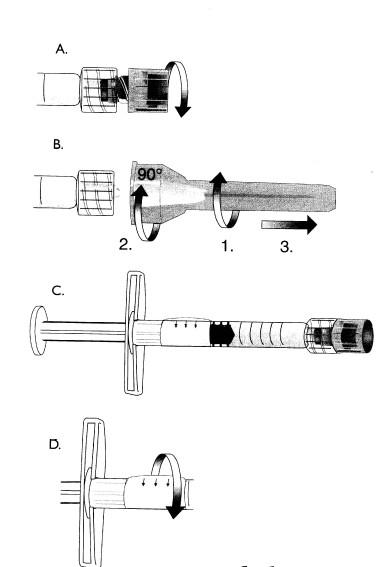
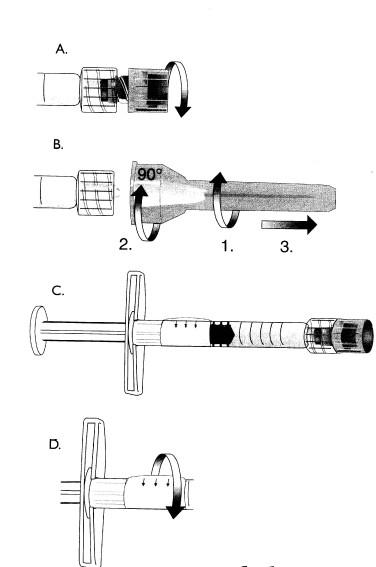
ASSEMBLY OF NEEDLE TO SYRINGE
It is important that the needle is properly assembled to the syringe. Improper assembly may result in separation of the needle and syringe during injection.Use the thumb and forefinger to hold firmly around both the glass syringe barrel and the luer-lock adapter.
Grasp the needle shield(or hub if using cannula)with the other hand. To facilitate proper assembly, both push and rotate firmly. See picture. Strict aseptic technique must be followed.
DOSAGE AND ADMINISTRATION
Before the treatment, the patient's suitability for the treatment and his/her medical history should be assessed.Patients should be informed of the indications of the product as well as its contraindications and potential adverse events before they are treated.The area to be treated must be thoroughly disinfected and the product should be injected under sterile conditions.
Keep the product at room temperature for 30 minutes prior to injection.Before injecting, press on the plunger of the syringe until a small drop is visible at the tip of the needle. Inject slowly to the dermis using the appropriate technique, either the linear threading technique or a series of punctual injections with the needle provided. The amount of the product to be injected perinjection site will depend on its severity. If blanching is observed, i.e. the skin turns a white color,the injection should be stopped immediately and the area massaged until it returns to a normal color. After the injection, massage lightly the correction site to conform to the contour of the surrounding tissues. Retouching a correction undertaken with the product should be immediately or a few weeks after the first injection.
CONTRAINDICATIONS
■ it is contraindicated in:
- case of known hypersensitive to sodium hyaluronate,
-.pregnant or breast-feeding women,
- young patients under 18 years old,
- patients presenting a general infection
- patients who tend to develop hypertrophic scarring.
■ Do not inject into the eye contours (eye circle or eyelids).
■ Do not inject into the blood vessels (intravascular).
■ Do not inject into areas presenting cutaneous problems of an inflammatory or infectious type
(acne,herpes…).
■ The product must not be used in association with laser therapy, chemica l peeling or dermal
abrasion.
PRECAUTION
General considerations relevante to injectable medical devices
·Injection procedures are associated with a risk infection. Aseptic technique and standard practice to prevent cross-infections are to be observed.
·Special caution should be exercised when treating areas in close proximity to permanent implant.
·knowledge of the anatomy of treatment site and special caution are required in order to avoid perforation Or compression of vessels and other vulnerable structures.
·Special caution should be exercised when treating areas with limited collateral circulation, due to increased Risk of ischemia.
·Special caution should be exercised in treating facial areas with limited soft tissue support or soft tissue cover.
Such as the periorbital area, to avoid formation of palpable lumps.
·Patients with pre-existing pigmented dark lower eye lid circles, thin skin and pre-existing tendency toward
Edema formation are not suitable candidates for treatment of the lower periorbital region.
·Do not use where there is active disease such as inflammation, infection or tumours, in or near the intended
Treatment site.
·injection procedures can lead to reactivation of latent or subclinical herpes viral infections.
·Patients who are using substances that affect platelet function, such as aspirin and non-steroidal
anti-inflammatory drugs may, as with any injection, experience increased bruising or bleeding at injection sites.
·patients with unattainable expectations are not suitable candidates for treatment.
·Do not use the product if package is damaged.
NEEDLE
It is used for infusion of medicines & Medical Instrument in the subcutaneous, intravenous or muscular tissues, or inhalation of body fluids. It is composed of cap, needle, and hub. It is a
disposable product..
SHELF LIFE AND STORAGE
Expiry date is indicated on each package. Shelf-life is 24months from the date of manufacture. Store between 2℃-25℃, and protect from direct sunlight and freezing.
PREPARATIONS
Check the expiration date of the product. Make sure that the package is not damaged or opened. Check if the needle is bad or contaminated with foreign matter.
INSTRUCTIONS
Remove the protective cover immediately before the procedure to prevent contamination of the needle. This product is single use, do not reuse. This product should be used by medical professionals.
SYMBOLS USED ON LABELING

![]() :Merry BioTechnology(YangZhou)Co.,Ltd
:Merry BioTechnology(YangZhou)Co.,Ltd
Add: B-6 ChangJiang Road225600,GaoyouJiangSu,China
Tel:86-571-56687275 Fax:86-571-56687273
Website:http://www.dermaxgel.com
EU Representative
Previous:Hyaluronic acid&cross-linked hyaluronic acid
Next:没有了!